JOURNAL SYNOPSIS
Title: Oxytocin Triggers Disruption of Mammary Epithelial Tissue and Stimulates Epithelial Cell Shedding
By : Muhammad Tegar K.K. S.Pt / First Expert Livestock Seed Supervisor
Journal: Journal of Mammary Gland Biology and Neoplasia (2018) 23:139–147
Published: June 8, 2018
Background and Research Objectives
The Mammary Gland consists of 2 somatic cell types: immune cells and mammary epithelial cells (MECs). Recently, it has been discovered that MECs are released due to shedding processes. The amount of shedding in milk affects milk production. In dairy cows, the daily shedding of MECs is approximately 390×10^6 or 0.007% of total MECs present in the udder. However, the mechanism regulating MEC shedding remains unknown.
In humans, there is a hypothesis that the pressure generated during the filling of secretory cells with milk synthesis as a result of breastfeeding leads to MEC shedding from ducts or luminal layers. Breastfeeding stimulates the brain to release Oxytocin (OT) into the bloodstream. OT is then captured by specific receptors present on myoepithelial cells, triggering their contraction. Contracting myoepithelial cells cause milk stored in alveoli to flow into mammary ducts and towards the nipple. This process occurs during both breastfeeding and machine milking.
In ruminants, especially dairy cows, a high rate of MEC shedding leads to damage in mammary epithelial tissue. The author hypothesizes that milking results in elevated OT activity in the mammary gland, causing myoepithelial cell contractions that trigger MEC shedding. In this experiment, the role of myoepithelial cell contractions in MEC shedding is explored by inducing or inhibiting myoepithelial activity during milking, using the OT receptor antagonist atosiban (Ato) or exogenous OT.
Materials and Methods
Eight peak-lactating FH cows were used in this experiment. Milking was conducted twice a day, at 7 a.m. and 5 p.m. Permanent catheters were placed in the jugular vein of experimental cows to deliver Ato and OT. The first treatment was the control with added OT, and the second treatment was with added OT and Ato. The experimental design is shown in the image below.
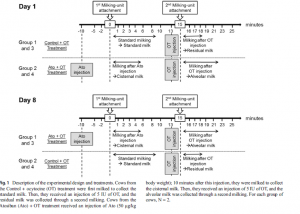
Figure 1. Experimental Design with OT and Ato Addition
Analysis of Milk Composition
Each 50 mL milk sample was measured for fat and lactose content using mid-infrared spectrophotometry and flow cytometry. Total protein was measured using the Kjeldahl method, and non-casein content was measured by adding 10% acetic acid and 1 M sodium acetate. Casein content was determined by subtracting non-casein from total protein.
Purification of Mammary Epithelial Cells (MECs)
To assess MEC content in milk, MECs were purified from milk using the immunomagnetic method. Flow cytometry was used for this immunomagnetic procedure.
Blood, Oxytocin, and Lactose Sampling
Blood samples were collected before, during, and after milking at -5, -2, 1, 2, 3, 4, 6, 8, 10, 13, 15, 16, 17, 18, 19, 21, 23, and 25 minutes using a milking machine. Monovette tubes coated with sodium heparin were used to collect blood samples for plasma OT and lactose concentration measurement. Plasma was separated by centrifugation for 15 minutes and stored at -20°C until analysis. Plasma OT was measured using the ELISA method.
RESULTS AND DISCUSSION
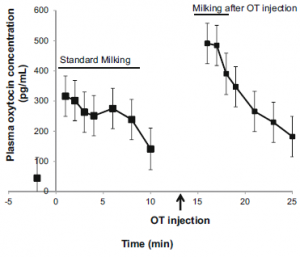
Figure 2. Plasma OT Content in Blood
Plasma OT levels increase shortly after milking begins and represent endogenous OT produced by normal physiological mechanisms. OT levels then gradually decrease until the 10th minute. At the 13th minute, additional OT is introduced through the catheter, leading to a sharp increase in plasma OT levels, reaching 491.1 ± 66.77, followed by a decrease until the 25th minute.



